5. PRMC Initial Submission
PRMC Requirements
Cancer-related studies must be reviewed by the Protocol Review and Monitoring Committee (PRMC). Cancer-Related Studies are studies where the objectives involve the diagnosis, prevention, screening, evaluation, treatment, or support of cancer patients and/or more than 30% of the patients involved in the study are likely to have an active cancer diagnosis. Cancer-related studies can include studies that involve obesity, smoking cessation, etc. PRMC conducts its reviews via OnCore, so it is mandatory that all studies requiring PRMC review have an OnCore record regardless of billing risk.
Submit to PRMC first!
Note that if PRMC review is required, you must submit the protocol for PRMC review before submitting it for IRB review. If you submit to IRB without PRMC approval, IRB will not review your submission until PRMC approval is obtained.
PRMC Requirements
In addition to the Yellow and Green highlighted fields that are required as part of the WashU Minimum Footprint, submissions to PRMC require additional fields to be completed. The protocol Regulatory Coordinator is required to enter fields highlighted in Purple on screenshots throughout this page at the time of Initial PRMC submission.
All modifications and continuing reviews for the study must also be submitted to PRMC concurrently with IRB submission. The only exceptions are study team updates (not involving a PI change) and study flyers/advertisements, which do not require PRMC review. PRMC instructions for modifications and renewals can be found here: 3. PRMC Submissions- Continuation, Change and Closure
Studies reviewed by PRMC require participant registration in OnCore in order to meet the requirements of the National Cancer Institute (NCI). This includes studies classified as ancillary/correlative. The New Studies Review Committee (NSR) will determine if your study requires individual level participant registration or summary level participant registration. The only exceptions are retrospective chart reviews and specimen analyses conducted under a waiver, which do not require participant registration in OnCore.
OnCore Guidance for:
- Individual level participant registration: D. Subject Management
- Summary level participant registration: 6. Summary Accruals
If you are unsure as to whether your study qualifies for individual level or summary accrual, please contact PRMC via email at protocoloffice@wudosis.wustl.edu.
If you do not have access to register participants in OnCore, please contact the OnCore Support Team at oncore@wustl.edu to request access.
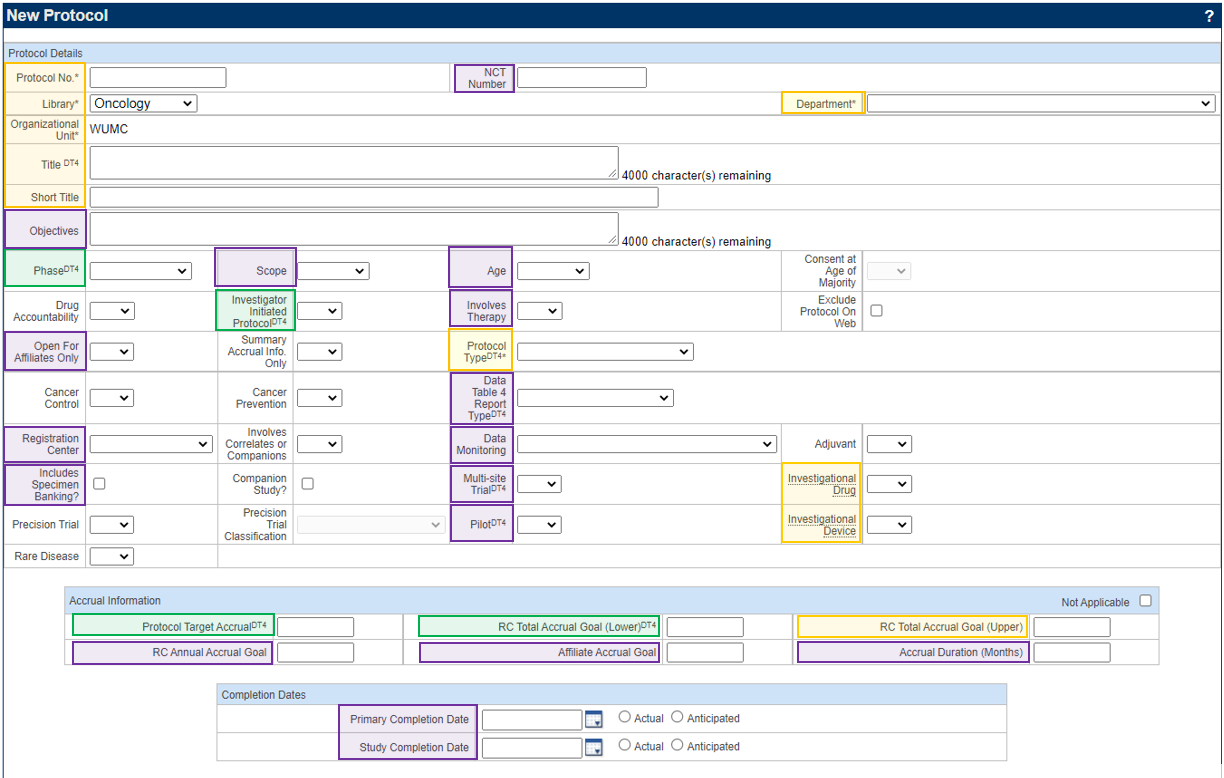
Protocol Details
Additional PRMC Required Fields (highlighted in Purple)
From the Menu, navigate to Protocols > PC Console
If you are creating a new protocol, click New Protocol in the vertical toolbar
If you are updating an existing protocol:
Type the HRPO number in the Select Protocol field and select from the drop down.
Click Update in the bottom right side of the page to open the fields for revisions.
- In the Protocol Details block, review or enter the following highlighted fields that make up the additional fields required for PRMC submission. Please make sure that the WashU Minimum footprint fields (instructions found here: 1. New Protocol Creation) are also completed at the time of PRMC submission. Please refer to PC Console > Main > Details tab below for Definitions.
- NCT number (if available)
- Objectives
- Scope
- Age
- Involves Therapy
- Open for Affiliates Only (this must be answered as NO or left blank in order for PRMC to review the submission)
- Data Table 4 Report Type
- Registration Center
- Data Monitoring
- Includes Specimen Banking?
- Companion Study
- Multi-site Trial
- Pilot
- RC Annual Accrual Goal
- Affiliate Accrual Goal
- Accrual Duration (Months)
- Primary and Study Completion Dates: These fields are used for clinicaltrials.gov tracking purposes ONLY. The dates are based on completion of primary and secondary outcome measures and are NOT based on when accrual is expected to be completed. If the study is an interventional investigator-initiated clinical trial, please contact Allison Creekmore at creekmorea@wustl.edu and she will register the trial to clinicaltrials.gov. She will also register any other types of investigator-initiated cancer-related clinical trials for investigators.
- When all required fields have been entered on the PC Console > Main > Details tab, click Submit.
- The Accrual Information fields must have values entered for any type of study, including retrospective chart reviews and specimen collection studies.
Yellow = Shell
Green = Minimum Footprint
Purple = PRMC Required
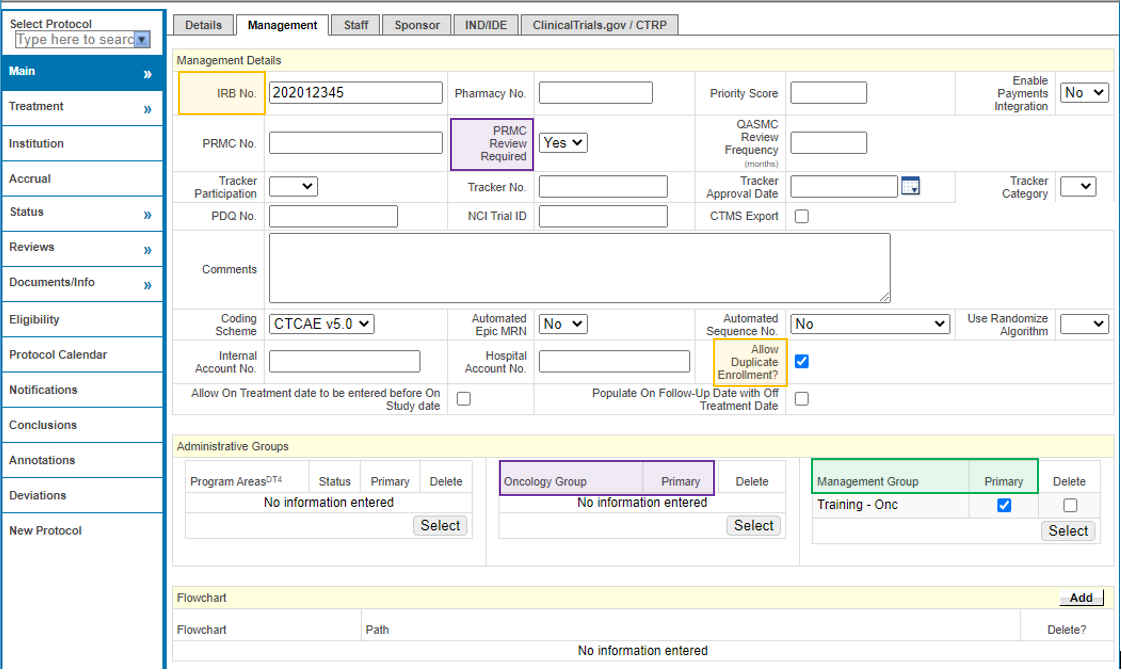
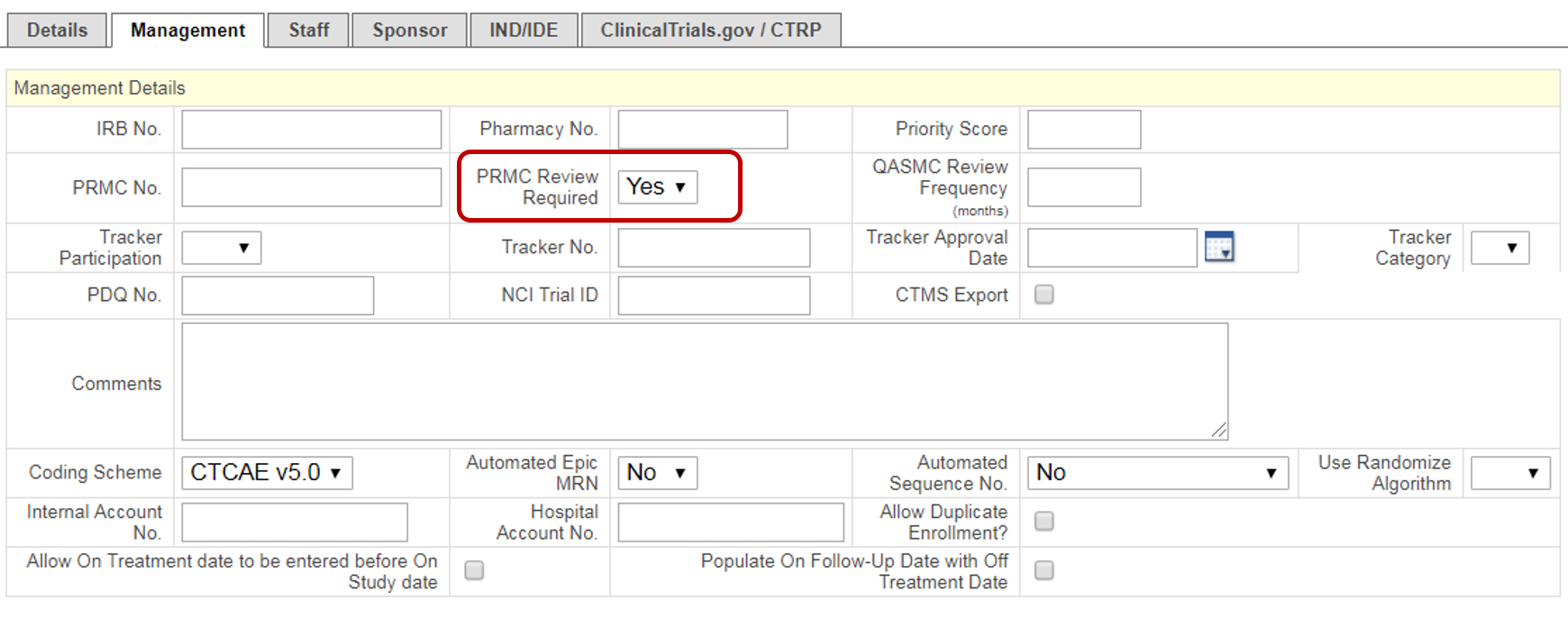
Management
Additional PRMC Required Fields (highlighted in Purple)
- From the PC Console, navigate to the Management horizontal tab from the Main tab in the vertical toolbar.
- Enter the following highlighted Purple fields that make up the additional fields required for PRMC submission. Please make sure that the WashU Minimum footprint fields (instructions found here: 1. New Protocol Creation) are also completed at the time of PRMC submission.
- PRMC Review Required
- Oncology Group
- When all required and desired fields have been entered on the PC Console > Main > Management tab, click Submit.
Yellow = Shell
Green = Minimum Footprint
Purple = PRMC Required
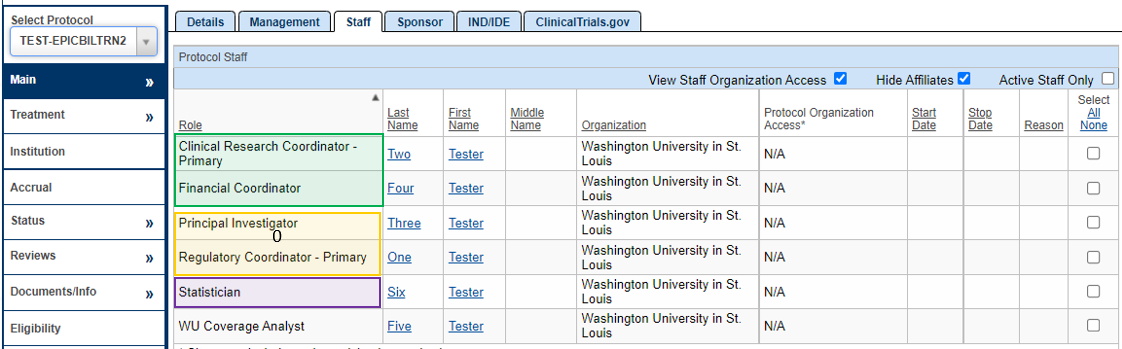
Staff
Additional PRMC Required Fields (highlighted in Purple)
- From the PC Console, navigate to the Staff horizontal tab from the Main tab in the vertical toolbar.
- Enter the following highlighted Purple fields that make up the additional fields required for PRMC submission. Please make sure that the WashU Minimum footprint fields (instructions found here: 1. New Protocol Creation) are also completed at the time of PRMC submission:
- Statistician
Yellow = Shell
Green = Minimum Footprint
Purple = PRMC Required
Sponsor
Additional PRMC Required Fields (highlighted in Purple)
The PC Console > Main > Sponsor page is where the protocol sponsor information is managed and displayed.
- From the PC Console, navigate to the Sponsor horizontal tab from the Main tab in the vertical toolbar.
- Please make sure that the WashU Minimum footprint fields (instructions found here: 1. New Protocol Creation) are also completed at the time of PRMC submission.
- Additional instructions for PRMC submission:
- For cooperative group studies, the Principal Sponsor will be the cooperative group.
- For WashU institutional studies, the Principal Sponsor will be Washington University UNLESS
- A grant was specifically obtained for the study, in which case the Federal funding agency (e.g. National Cancer Institute, National Institutes of Health, etc.) will be the Principal Sponsor.
- The principal investigator is a Barnes employee and the study doesn’t have a federally-funded grant that was specifically obtained for the study, in which case BJC Healthcare will be the Principal Sponsor.
- For secondary institutional studies, the Principal Sponsor will be the academic organization that is leading the study.
- For industry studies (e.g. studies led by a pharmaceutical company), the Principal Sponsor will be the pharmaceutical company funding the study.
- After addition of all sponsors and their roles, press Submit.
- NOTE: The New Studies Review Committee (NSR) will review all new studies for accuracy on sponsor listings. If any questions, please contact the PRMC office at protocoloffice@wudosis.wustl.edu.
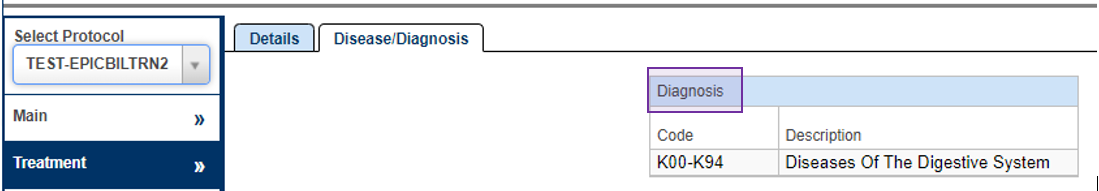
Disease/Diagnosis
Additional PRMC Required Fields (highlighted in Purple)
- From the PC Console, navigate to the Treatment tab in the vertical toolbar and then navigate to the Disease/Diagnosis tab in the horizontal toolbar.
- In the Disease/Diagnosis block, click Update.
- Click Select and check the box next to all applicable Data Table 3 sites and then click Add.
- After addition of all applicable Data Table 3 sites, press Submit.
- Please refer to instructions/examples for selecting the appropriate Data Table 3 sites here: DT3 Disease Codes
Yellow = Shell
Green = Minimum Footprint
Purple = PRMC Required
Additional PRMC Instructions
The PC Console > Institution page is used to display, add, and delete the institutions and study sites that are participating in the protocol. All participating institutions who will be reporting their enrollments in OnCore are required to be added. For cancer-related investigator-initiated studies, all participating institutions are required to be added because the NIH requires WashU to report the enrollments of all participating institutions.
- Please make sure that the WashU Minimum footprint fields (instructions found here: 1. New Protocol Creation) are also completed at the time of PRMC submission.
Creating the PRMC Submission
Once the new protocol shell has been created and all Minimum Footprint and PRMC required fields are complete (see 1. New Protocol Creation and PRMC Requirements instructions above), the protocol may now be submitted for PRMC review.
- From the Menu, navigate to Protocols > PC Console and select the Management horizontal tab from the Main vertical tab.
- Change the PRMC Review Required drop down to Yes. Make sure that the protocol shell is complete before taking this action!
When you click Submit, a pop-up will appear stating: Do you want to create an ePRMS initial submission?
Click OK. This creates a submission for the protocol and sends it to the ePRMS Submissions Console.
Be sure to click OK!
Even if you change the drop down to Yes, if you do not click OK on the pop-up, the submission will not be created. If this occurs, change the drop down back to No or blank and click Submit; change the drop down back to Yes and click Submit again, then click OK on the pop-up.
PRMC Create Initial Review Link
When the "PRMC Review Required" is set to Yes, indicates that the protocol can't be opened to accrual until there is an approved PRMC Initial Review entered in OnCore. Additionally, a Create Initial Review link appears under this field (regardless of how it is set) in update mode when the following conditions are met:
- The protocol has a library of Oncology.
- The protocol doesn't already have a PRMC Initial Review.
- The protocol's status is not Closed to Accrual, IRB Study Closure, Abandoned, or Terminated.
- You have the Regulatory access role.
When you click this link, you are directed to a draft of the Initial Review in the ePRMS > Submission Console. From here, skip to step 3 of Submitting to PRMC below.
Submitting to PRMC
- From the Menu, navigate to ePRMS > Submission Console.
- If the submission is not currently displayed, search for it by entering the protocol number in the Submission No./Protocol No./PRMC No. field. Unlike many fields in OnCore, this is not a predictive field and will not populate as you type. You must enter the number exactly and then click enter. A list of submissions will appear from which you can click the hyperlinked submission.
- Scroll down to the bottom of the page and click Update.
- In the Institutions block, confirm that only participating study sites are selected.
- Complete the Competing Protocols field. Competing protocols are treatment protocols that deal with the same disease with partial or complete overlap of eligibility criteria.
- If there are 1 or more competing protocols:
- Search within the Protocol No field for any competing protocols. This is not a predictive field. When you have entered the full or partial protocol number, click the arrow to generate a pop-up list of all matching protocols.
- Next, click the hyperlinked Protocol No. for the applicable completing protocol. Repeat this process until all competing protocols have been added.
- List out all competing protocols, in order of prioritization as they would be offered to/considered for potential patients.
- If there are no competing protocols, indicate as such by checking the box next to No Completing Protocol.
- If there are 1 or more competing protocols:
- Upload the PRMC required documents to the Documents block. For a list of PRMC required documents, please reference PRMC Submission Forms and Instructions.
- When the updates have been made and you have confirmed that all other information entered is correct, click Save.
- When you are ready to submit to PRMC, click Send.
- Your submission will be reviewed and a notification will be sent to you when a decision is made. Do not proceed with IRB submission until the study has received PRMC approval.
Responding to PRMC Notifications
If your PRMC submission is Contingent or Deferred, you will need to respond to the query in order to move the submission forward. The study team will receive notification of the status and the details of the query.
- From the Menu, navigate to ePRMS > Submission Console.
- If the submission is not currently displayed, search for it by entering the protocol number in the Submission No./Protocol No./PRMC No. field. This is not a predictive field and will not populate as you type. You must enter the number exactly and then click enter. A list of submissions will appear from which you can click the hyperlinked submission.
- To respond to the query:
- Make all necessary changes to the submission per the query details.
- Scroll to the bottom of the page and click Update.
- If the query is due to missing or incomplete documentation, attach all relevant documents.
- Make any other adjustments to the information on the page per the query details.
- Click Save and Close.
- Respond to the query and resend the submission.
- At the bottom of the page, click Query Detail.
- In Query Detail block, click Edit.
- In Response text box, list the changes you made.
- Click Submit and Respond.
- Make all necessary changes to the submission per the query details.
- As with the initial submission, you will receive notification of committee decision.
- If approved, you may now proceed with IRB Submission.
- If contingent or deferred, repeat the steps outlined above.
Definitions
PC Console > Main > Details tab
The PC Console > Main > Details tab is where basic information about a protocol is recorded. This page is the first page populated when creating a new protocol in OnCore.
- Protocol No.: This is the main identifier for a protocol and will appear in reports and searches.
- OnCore integrates with myIRB to link the study across the two systems, allowing select review and status information to flow from myIRB into OnCore. Therefore, you must enter the protocol number as assigned by HRPO in the Protocol No. field.
- If you create the protocol shell in advance of IRB submission and you do not yet have a HRPO number assigned, please enter the PRMC number (for oncology studies) or the short title on your HRPO application.
- NCT Number: This is the clinicaltrials.gov number that is assigned at the time of clinicaltrials.gov registration. Add the NCT number if available. If the study is a cancer-related (submitted to PRMC) interventional investigator-initiated clinical trial, please contact Allison Creekmore at creekmorea@wustl.edu and she will register the trial to clinicaltrials.gov. She will also register any other types of investigator-initiated cancer-related clinical trials for investigators.
- Library: The library you select determines which set of configurations are made accessible for the protocol in OnCore. Select Oncology if PRMC review is required. For guidance on which studies require PRMC review, please visit the PRMC website.
- Department: In myIRB, Department is defaulted based on the PI; this may differ from Management Group. Department identifies the group responsible for managing study financials, determines privileges, and routes information for financial reports.
- Organizational Unit: This will default to WUMC as there is only 1 Organizational Unit in OnCore.
- Title: Identifies the full-length name of the protocol. The title populates to other pages within the OnCore application and is displayed in some reports.
- Short Title: An abbreviated version of the protocol title with 100 character maximum, the short title populates throughout OnCore and in some reports
- Through the OnCore-Epic integration, the short title in OnCore populates as the study title in Epic.
- Objectives: The full list of objectives from the protocol.
- Phase: For interventional clinical trials evaluating drugs/biologics/vaccines, select the accurate Phase. For interventional clinical trials evaluating radiation therapy, devices, or assays select N/A. For expanded access studies, select N/A. For observational, ancillary, correlative, not applicable, or single patient DT4 studies, select N/A.
- Scope: Choose National if the study is taking place at other sites in addition to WashU. Choose Local if the study is only taking place at WashU.
- Age: If the study is enrolling participants who are ≥ 18 years of age, choose Adults. If the study is enrolling participants who are < 18 years of age, choose Children. If the study is enrolling participants who are both Adults and Children, choose Both.
- Consent at Age of Majority: When Adults is chosen for Age, this field is grayed out. If Children or Both is chosen for Age, this field opens up. If a child < 18 years of age is enrolled and then they turn 18 and become an adult (at the age of majority) while they are a participant, they are to be consented into the study using the main consent form and not just the assent form. This field would be marked as “yes” if there is a formal consent for the study.
- Investigator Initiated Protocol: This field indicates if the Principal Investigator for the study initiated the protocol.
- Yes: The trial originated and/or is sponsored at WashU
- No: The trial originated and/or is sponsored outside of WashU
- Involves Therapy: Used to indicate a therapeutic trial. If set to “Yes” a subject that is actively enrolled in the trial is prevented from being registered on another therapeutic trial. Do not use “N/A”.
- Exclude Protocol on Web: PRMC will complete this field for studies in the Oncology library.
- Open For Affiliates Only: This field must be marked as “No” or left blank in order for PRMC to review the submission.
- Summary Accrual Info. Only: For studies in the Oncology library, the New Studies Review Committee completes this field. Contact the Siteman Cancer Center (SCC) Protocol Office at protocoloffice@wudosis.wustl.edu for questions regarding this field.
- Protocol Type: Indicates the type of protocol, and is used in reports.
- Basic Science: designed to examine the basic mechanisms of actions (e.g., physiology, biomechanics) on an intervention.
- Device Feasibility: an intervention of a device product is being evaluated in a small clinical trial (generally fewer than 10 participants) to determine the feasibility of the product; or a clinical trial to test a prototype device for feasibility and not health outcomes.
- Diagnostic: evaluating one or more interventions aimed at identifying a disease or health condition
- Health Services Research: designed to evaluate the delivery, processes, management, organization, or financing of health care
- Other: not identified by any other categories, if the study is an expanded access study, the protocol type will be classified as “other”
- Prevention: designed to assess one or more interventions aimed at preventing the development of a specific disease or health condition
- Screening: designed to assess or examine methods of identifying a condition (or risk factor for a condition) in people not yet known to have the condition (or risk factor)
- Single Patient: Only select this if the study is being conducted on one patient.
- Supportive Care: designed to evaluate one or more interventions where the primary intent is to maximize comfort, minimize side effects, or work against a decline in participant health or function (not curative).
- Treatment: designed to evaluate one or more interventions for treatment of a disease, syndrome or condition (therapeutic)
- Data Table 4 Report Type: (Oncology Library only) Indicates the type of study and is used in reports. The New Studies Review Committee will review for accuracy:
- Ancillary/Correlative:
- Ancillary: Hypothesis-driven studies that are stimulated by, but are not a required part of, a main clinical trial/study, and that utilize patient or other resources of the main trial/study to generate information relevant to it. Ancillary studies must be linked to an active clinical research study and should include only patients accrued to that clinical research study. Only studies that can be linked to individual patient or participant data should be reported.
- Correlative: Hypothesis-driven laboratory-based studies using specimens to assess cancer risk, clinical outcomes, response to therapies, etc. Only studies that can be linked to individual patient or participant data should be reported.
- Interventional: Hypothesis-driven studies where individuals are assigned prospectively by an investigator based on a protocol to receive specific intervention(s). The participants may receive diagnostic, treatment, behavioral, or other types of intervention(s). The assignment of the intervention(s) may or may not be random. The participants are followed so that effects of the intervention(s) on biomedical and/or health outcomes are evaluated.
- Not Applicable: Not identified by the other categories.
- Observational: Hypothesis-driven studies that focus on cancer patients and healthy populations and involve no prospective intervention or alteration in the status of the participants. Biomedical and/or health outcome(s) are assessed in pre-defined groups of participants. The participants in the study may receive diagnostic, therapeutic, or other interventions, but the investigator of the observational study is not responsible for assigning specific interventions to the participants of the study.
- Ancillary/Correlative:
- Registration Center:
- Research Center: Select if the study is sponsored by WashU.
- External: Select if the study has an industry sponsor or if another institution is the principal sponsor.
- Data Monitoring: (Use for Oncology Only) Indicates the party responsible for monitoring the protocol data. For lines below denoted with QASMC: Audits are required for any institutional (WashU and non-WashU) and interventional studies. Check with the coordinating center to determine what tasks they will be performing for the study, and this will guide what category below to select.
- External: Pharmaceutical (industry) sponsor or Cooperative Group sponsor
- PI: The Principal Investigator monitors the data for non-interventional studies (e.g. Banking/Registry studies and Retrospective Chart Reviews)
- QASMC - External Audit, External DSM : Study is sponsored by another institution (the coordinating center) which will be conducting the audits and will also be preparing the data safety monitoring (DSM) reports for the WashU patients. These reports are then submitted to SCC’s Quality Assurance and Safety Monitoring Committee (QASMC) for review.
- QASMC – External Audit, Internal DSM: Study is sponsored by another institution (the coordinating center) which will be conducting the audits of our patients, but the DSM reports are not being provided by the coordinating center and will be prepared by the WashU study team.
- QASMC – External Audit, Internal DSMB: Study is sponsored by another institution (the coordinating center) which will be conducting the audits, and an internal WashU Data Safety Monitoring Board (DSMB) is needed.
- QASMC – Internal Audit, External DSM: If WashU is the primary sponsor (the coordinating center) or WashU is the secondary center and our patients are not already being audited by the main coordinating center, then the WashU QASMC will conduct the audits and the other institution will only be preparing the DSM reports.
- QASMC – Internal Audit, Internal DSM: If WashU is the primary sponsor (the coordinating center) or WashU is the secondary center and our patients are not already being audited by the main coordinating center, then the WashU QASMC will conduct the audits, and the DSM reports are not being provided by the coordinating center and will be prepared by the WashU study team.
- QASMC – Internal Audit, Internal DSMB: If WashU is the primary sponsor (the coordinating center) or WashU is the secondary center and our patients are not already being audited by the main coordinating center, then the WashU QASMC will conduct the audit. A WashU Data Safety Monitoring Board (DSMB) is required for any institutional clinical trial where WashU is the primary center, there is treatment involved, AND one of the following: the target accrual is > 300, it is a double blinded treatment trial, the trial is multicenter, the trial is Phase III, or the study is “high risk” as determined by PRMC.
- QASMC – No audit, Internal DSM only: Low risk studies where no auditing is required but the WashU study team prepares the DSM reports (e.g. Imaging studies)
- Includes Specimen Banking?:
- Yes: The study is banking specimens
- No: The study is not banking specimens
- Companion Study?: Select this box if the protocol is a companion to another study, i.e. the data and/or specimens to be used for the current study are gathered from a previously-approved study
- Multi-site Trial:
- Yes: There are other sites besides WashU participating in the study
- No: Only WashU is participating in the study
- Investigational Drug: This field sets the value of the Investigational Drug field in the IND/IDE horizontal tab in the PC Console Main tab.
- Yes: There is an IND or IND exemption.
- No: There is no IND.
- N/A: IND designation does not apply.
- Yes: There is an IND or IND exemption.
- Investigational Device: This field sets the value of the Investigation Device field in the IND/IDE horizontal tab in the PC Console Main tab.
- Yes: There is an IDE.
- No: There is no IDE.
- N/A: IDE designation does not apply.
- Pilot :
- Yes: The study is a pilot study and the protocol indicates it is a pilot study.
- No: The study is not a pilot study and the protocol does not indicate it is a pilot study.
- Rare Disease: No need to complete this- Siteman Protocol office will use Annotations page to document Rare Disease.
- Protocol Target Accrual: The total number of subjects to enroll for the whole study. The target accrual number entered displays in the top header of most screens within OnCore and will populate in some reports.
- RC Total Accrual Goal (Lower): RC stands for “Research Center” which is WashU. The minimum number of subjects planned to be enrolled at WashU. The Low Accrual Report utilizes this field. This number should never be lower than the accrual to date. Usually this field is only lower than the Protocol Target Accrual if it is a Phase I or Phase I/II study.
- RC Total Accrual Goal (Upper): RC stands for "Research Center" which is WashU. This field is populated with the maximum number of subjects the IRB has approved to be consented at WashU.
- RC Annual Accrual Goal: This is the number of subjects expected to be enrolled each year and is calculated using the RC Total Accrual Goal (Lower) and Accrual Duration (Months).
- Affiliate Accrual Goal: The number of subjects to enroll at participating sites (not including WashU). The Affiliate Accrual Goal plus the RC Total Accrual Goal (Lower) should equal the Protocol Target Accrual.
- Accrual Duration (Months): The estimated number of months it will take to reach the Protocol Target Accrual. For retrospective chart review or specimen studies, this will be the estimated number of months it will take to collect all of the data needed for the study.
- Primary Completion Date: Estimated date that the final subject will receive an intervention for the purposes of final collection of data for the primary outcome measure(s) (use Anticipated).
- This field is only required for interventional investigator-initiated trials.
- Study Completion Date: Final date that data is expected to be collected for the remaining outcome measure(s) (use Anticipated).
- This field is only required for interventional investigator-initiated trials.
PC Console > Main > Management tab
The PC Console > Main > Management tab is where administrative information about the protocol is tracked. Some departments may have their own requirements beyond the Minimum Footprint requirements.
- IRB No.: Like Protocol No. in the Details page, IRB No. is searchable in Select Protocol fields
- For studies using HRPO, this number is the same as the protocol number.
- For studies using an external IRB, this is the IRB number as assigned by the IRB of record.
- PRMC Review Required:
- Yes: The study is a cancer-related study
- No: The study is not a cancer-related study
- NCI Trial ID: (Oncology library only) This field is for CTRP tracking purposes only and will be completed by the Siteman Cancer Center CTRP Coordinator.
- Automated Sequence No.: If marked Yes, subject sequence numbers will be automatically generated based on rules you set up. Once a subject becomes eligible and is put On Study in the On Study tab of the Subject Console, the next available sequence number will be assigned to that subject and will auto-populate in the Sequence Number field. If the study has different accrual goals based on arm or subject demographic factors such as age, sex, ethnicity, etc., different accrual goals can be set by selecting Stratified Block. Complete instructions can be found on the Stratification Blocks page.
- Internal Account No: This is required for the Workday-OnCore integration for Financials. After the study team creates the project in Workday, the Center for Clinical Studies (CCS) will review, approve and enter the Workday Project No. as the Internal Account No. This needs to be entered prior to the first invoice creation in OnCore.
- Allow Duplicate Enrollment:
- Yes: If the study allows previously screen failed subjects to re-screen and re-enroll, this field must be set to yes in order to allow a single subject to be enrolled on the protocol multiple times.
- No: When set to no, a single subject may only be enrolled on the protocol once. If you attempt to enroll a subject who has already been added to the protocol, the system will not allow this action.
- Yes: If the study allows previously screen failed subjects to re-screen and re-enroll, this field must be set to yes in order to allow a single subject to be enrolled on the protocol multiple times.
- Management Group: The team responsible for enrolling/managing study participants and/or collecting data for primary research endpoints. This field defines access privileges and shows up in reports. Select all that apply and select one Primary.
- Program Areas: (Oncology Library Only) These selections will be used in the Data Table 4 Report and will be completed by the New Studies Review Committee.
- Oncology Group: (Oncology Library Only) These are internal reporting groups. The Oncology Group marked as Primary will become the default value for the Oncology Group field on the Subject Console > On Study tab
PC Console > Main > Staff
The PC Console > Main > Staff tab is where staff members associated with the protocol are managed.
- Protocol Creator: This staff role is automatically added as the user who creates the protocol shell.
- Principal Investigator: The WashU or BJC PI for the study.
- Regulatory Coordinator - Primary: The primary staff member responsible for regulatory submissions for the protocol. There should be only one person listed in this role.
- Clinical Research Coordinator - Primary: The primary staff member responsible for subject management for the protocol. There should be only one person listed in this role.
- Financial Coordinator: The staff member(s) responsible for approving the billing grid and completing the Budget Team Signoff on the calendar. Also, may be responsible for OnCore Financial activities, like budgeting and invoicing.
- The following roles may listed in your Protocol Staff list. These are added by the CRBS Admin for the purpose of Coverage Analysis. Do not remove or change any of these entries.
- BJC Coverage Analyst
- WashU Coverage Analyst
- CRBS Admin
PC Console > Main > Sponsor tab
The PC Console > Main > Sponsor page is where the protocol sponsor information is managed and displayed.
- Role(s): roles that the sponsor will play for the protocol.
- Agent Source - will provide the drug/agent.
- CRO (Contract Research Organization) - an organization contracted by the sponsor to perform research functions.
- Data Analysis - will conduct data analysis.
- Design - responsible for trial design.
- Funding Source - provides funding
- Principal Sponsor: Sponsor who is funding the study. This is the sponsor that will appear in the protocol header.
- Guidelines for studies in the Oncology Library:
- For cooperative group studies, the Principal Sponsor will be the cooperative group.
- For WashU institutional studies, the Principal Sponsor will be:
- The Federal funding agency (e.g. National Cancer Institute, National Institutes of Health, etc.) if the grant was specifically obtained for the study.
- BJC Healthcare if the principal investigator is a Barnes employee and the study doesn’t have a federally-funded grant that was specifically obtained for the study.
- For all other studies, Washington University will be the Principal Sponsor.
- For secondary institutional studies, the Principal Sponsor will be the academic organization that is leading the study.
- For industrial (pharma) studies, the Principal Sponsor will be the pharmaceutical company funding the study.
- The New Studies Review Committee (NSR) will review all new studies for accuracy on sponsor listings. If any questions, please contact the PRMC office at protocoloffice@wudosis.wustl.edu.
- Guidelines for studies in the Oncology Library:
- Sponsor Protocol No.: The protocol number assigned by the sponsor; this must match information submitted to myIRB. For Investigator Initiated Trials, use the HRPO number. This field is optional, but departments may have specific requirements.
Need more help? Contact the OnCore Support Team: oncore@wustl.edu